Patient-Reported Outcomes in Scleroderma: Why it Matters

Scleroderma patients are the experts in how they feel and function. They are the only ones capable of describing first-hand the long list of symptoms associated with scleroderma, including Raynaud’s, skin changes, gastrointestinal problems and breathlessness to name a few, and how these impact their day-to-day life. They also have lived experience with the disfigurement and the complex psychosocial impacts of this disease.
The growing recognition that patients have unique experiences and values has resulted in a paradigm shift in the 21st century from a traditional paternalistic model of medicine with the physician deciding what is in the best interest of the patient to one of patient-centered care where patients are at the center of the health care continuum and their specific needs and goals are the driving force behind all health care decisions. Patient-centered care paved the way for patient-centered research.
 |
||
|
PROS AND PROMS Patient-reported outcomes (PROs) are defined as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else”. (1) PROs is an umbrella term that covers not only symptoms but also function, health-related quality of life (HRQL), satisfaction and so on. They capture patient experiences of disease that clinical assessments, blood tests, imaging (eg. CT scans and cardiac echocardiograms) and biopsies cannot. PROs have in fact become a central part of drug approvals as regulatory agencies including Health Canada, the US Food and Drugs Administration and the European Medicines Agency now require data not only on survival (and other measures of biological effectiveness) but on how patients “feel and function” when considering applications for new drug approvals. Patient-reported outcomes measures (PROMs) are the questionnaires that measure PROs in a standardized way. PROMs are either generic, in that they can be used across various health conditions, or specific, in that they aim to capture aspects of disease that are particular to a certain condition. |
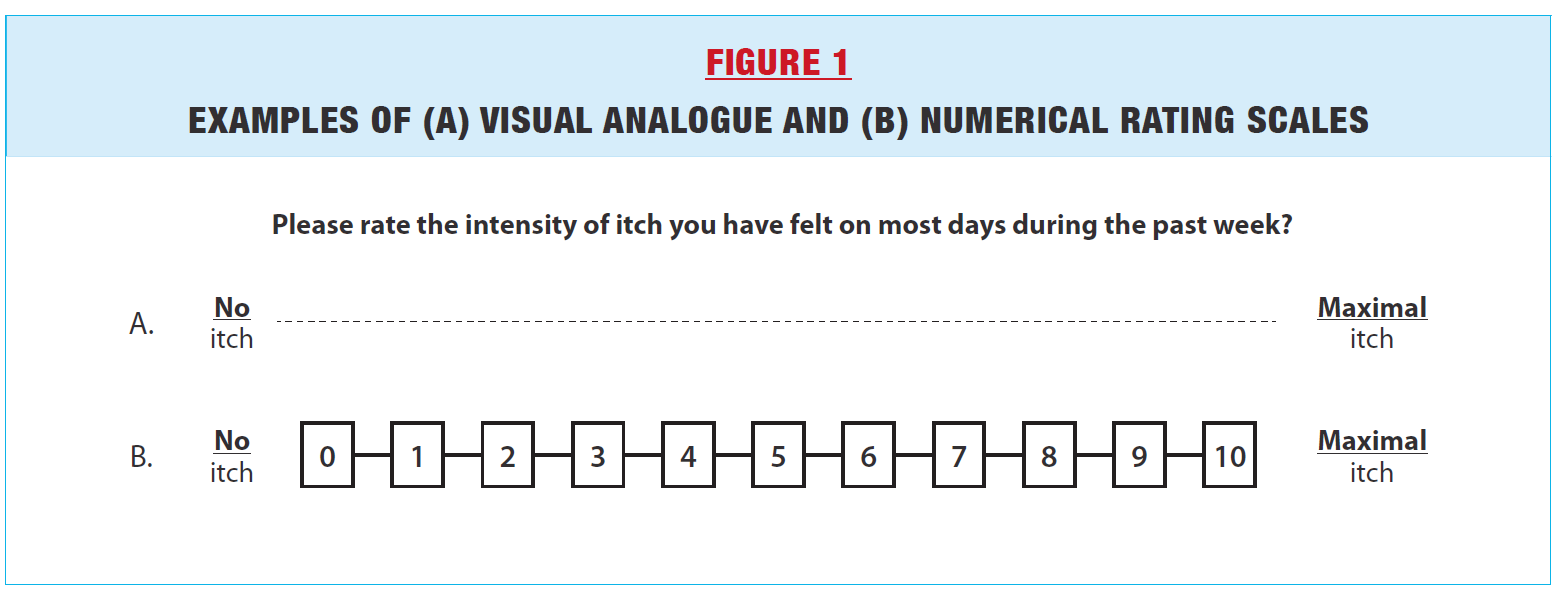
GENERIC PROMS The three most commonly used generic PROMs in scleroderma are patient global assessments, the Health Assessment Questionnaire (HAQ) and the Medical Trust Short-Form 36 (SF-36). The Patient-Reported Outcomes Measurement Information System-29 (PROMIS-29) is emerging as a novel generic PROM for scleroderma. Patient Global Assessments of disease generally ask patients to rate their overall health over a given period of time (eg. past week, past month), for example using a 10 cm visual analogue scale or numerical rating scale ranging from 0 to 10 (Figure 1). Studies have shown that scleroderma patients often rate disease severity as worse compared to physician ratings, suggesting that patient ratings capture different, possibly more complex psychosocial factors that physicians do not take into account. (2) On the other hand, in a disease as heterogeneous as scleroderma, with some patients having predominantly skin, others gastrointestinal and others still respiratory symptoms, Patient Global Assessments lack granularity. |
|
The HAQ is one of the earliest PROs used in rheumatic diseases. It is a questionnaire developed in 1980 to assess functional ability in patients with rheumatoid arthritis. It includes 20 questions in 8 categories (dressing and grooming, arising, eating, walking, hygiene, reach, grip, and performing activities). The patient rates his/her difficulty over the past week in performing the specific tasks in each category as none, mild, moderate or severe difficulty. The final score ranges from 0 (no disability) to 3 (severe disability). Although the HAQ is widely used, including in scleroderma, it has some limitations. It focuses mainly on musculoskeletal function and does not take into account the panoply of other functional limitations encountered in scleroderma, for example from breathlessness, gastrointestinal symptoms or body image distress. Also, there are concerns that even as a measure of musculoskeletal function, it may be outdated. For example, it does not include hand function such as keyboarding or using a cell phone, which is often compromised in scleroderma.
The SF-36 is possibly the most widely used generic measure of health-related quality of life. It is composed of 36 questions that can be grouped into 8 domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. The scores of the domains and summary scores are standardized with means of 50 and standard deviations of 10. Lower scores represent worse health-related quality of life. A systematic review of the literature reported that the Physical component summary score of the SF-36 was more than 1 standard deviation below the general population (38.3; 95% credible interval 35.2, 41.5).(3) This translates into saying that, on average, scleroderma patients are among the 15% of the population with the worst physical health-related quality of life. The SF-36 has the advantage that it has been used in many diseases and allows for cross-disease comparisons. The impairments in SF-36 in scleroderma are worse than the general population and as high if not higher than other more common chronic diseases namely heart disease, lung disease, hypertension, diabetes, and depression.(4) This provides valuable information to advocate for health care resources for scleroderma, which remains relatively unknown among the public and health care decision-makers.
PROMIS-29 is a more recent generic measure of health-related quality of life developed by the National Institutes of Health (https://www.healthmeasures.net). It has the advantage of including a domain for sleep, which is often impaired in scleroderma. A study from the Scleroderma Patient-centered Intervention Network (SPIN) cohort showed that joint contractures and gastrointestinal symptoms were the strongest predictors of worse PROMIS-29 scores, (5) providing valuable priorities for further scleroderma research with the potential to improve health-related quality of life.
 |
||
|
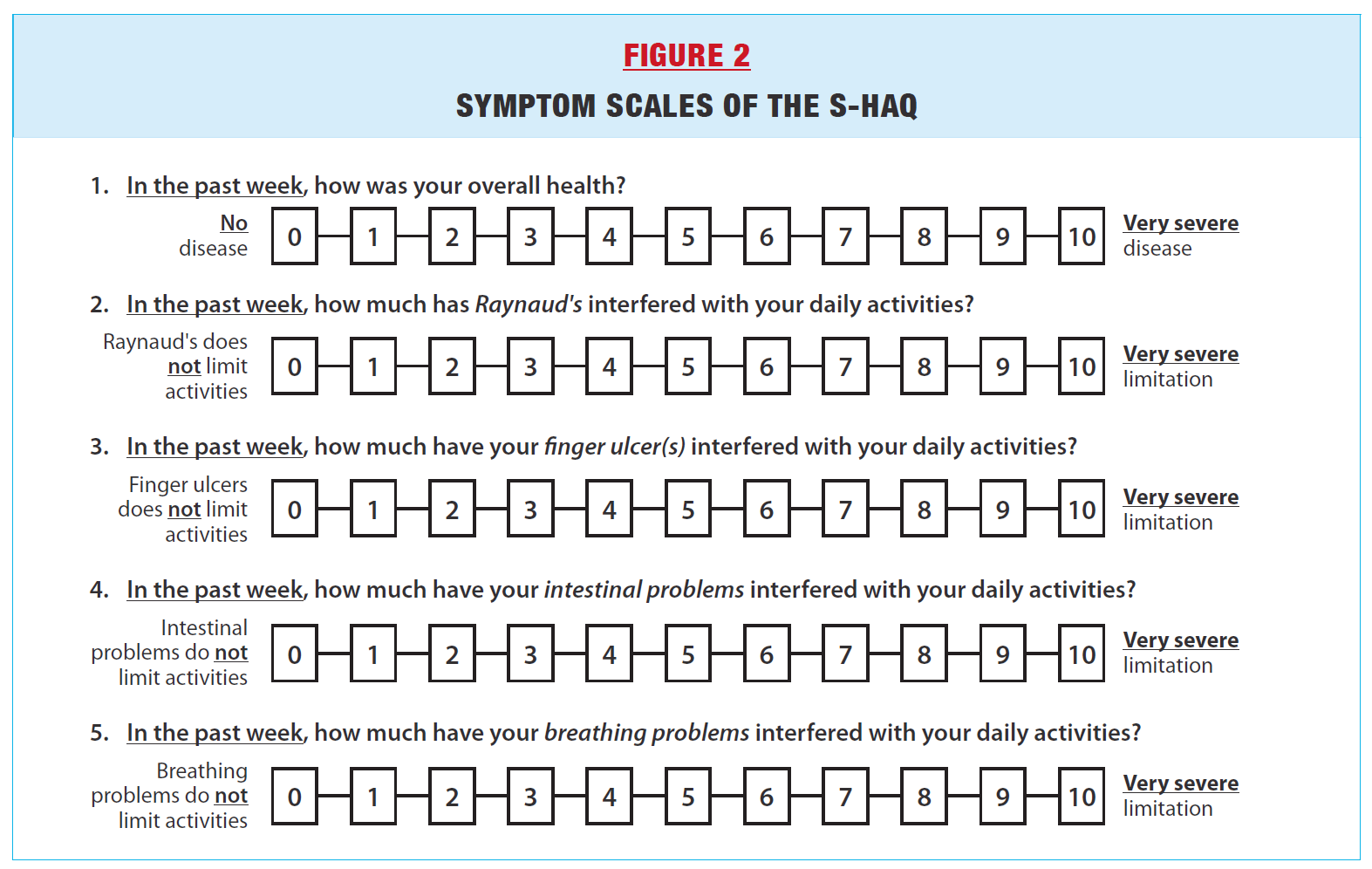
PROMS SPECIFIC FOR SCLERODERMA As mentioned above, the HAQ is a generic measure of musculoskeletal function. To supplement the HAQ with scleroderma- specific content, Steen and Medsger proposed in 1997 to add 5 symptom scales asking patients to rate how much overall disease, Raynaud’s phenomenon, finger ulcers, breathing and gastrointestinal problems interfere with daily activities (Figure 2).(6) Higher scores represent worse symptoms. The addition of these symptom scales to the HAQ, which is commonly referred to as the Scleroderma-HAQ or S-HAQ, was an important first step towards developing scleroderma-specific PROs and capturing important information from the patient’s perspective. The extent of skin disease has traditionally been measured by the modified Rodnan skin score (mRSS), which is an assessment of skin thickness ranging from 0-3 in 17 areas of the body performed by a physician (range 0-51, with higher scores representing worse skin thickening). The mRSS is commonly used as a primary endpoint in clinical trials as it has been shown to predict internal organ involvement and mortality in scleroderma. However, it does not |
capture the subjective experience of skin changes in scleroderma. The Scleroderma skin patient-reported outcome (SSPRO) was recently developed with the input of patients to capture the patients’ experiences of skin-related health changes in scleroderma.(7) It is an 18-item patient questionnaire that assesses 4 domains: physical effects, physical limitations imposed by skin tightness, emotional effects and social effects, with each item being graded from 0 to 6 (range 0-108, with higher scores representing worse assessments). The SSPRO has been shown to capture how a patient feels and functions (by correlating it with traditional PROMs). Interestingly, in one clinical trial, the SSPRO correlated only moderately with the mRSS, (8) indicating that there are likely additional factors beyond assessments of skin severity by physicians that shape the experience of SSc patients regarding their skin condition.
|
|
 |
||
|
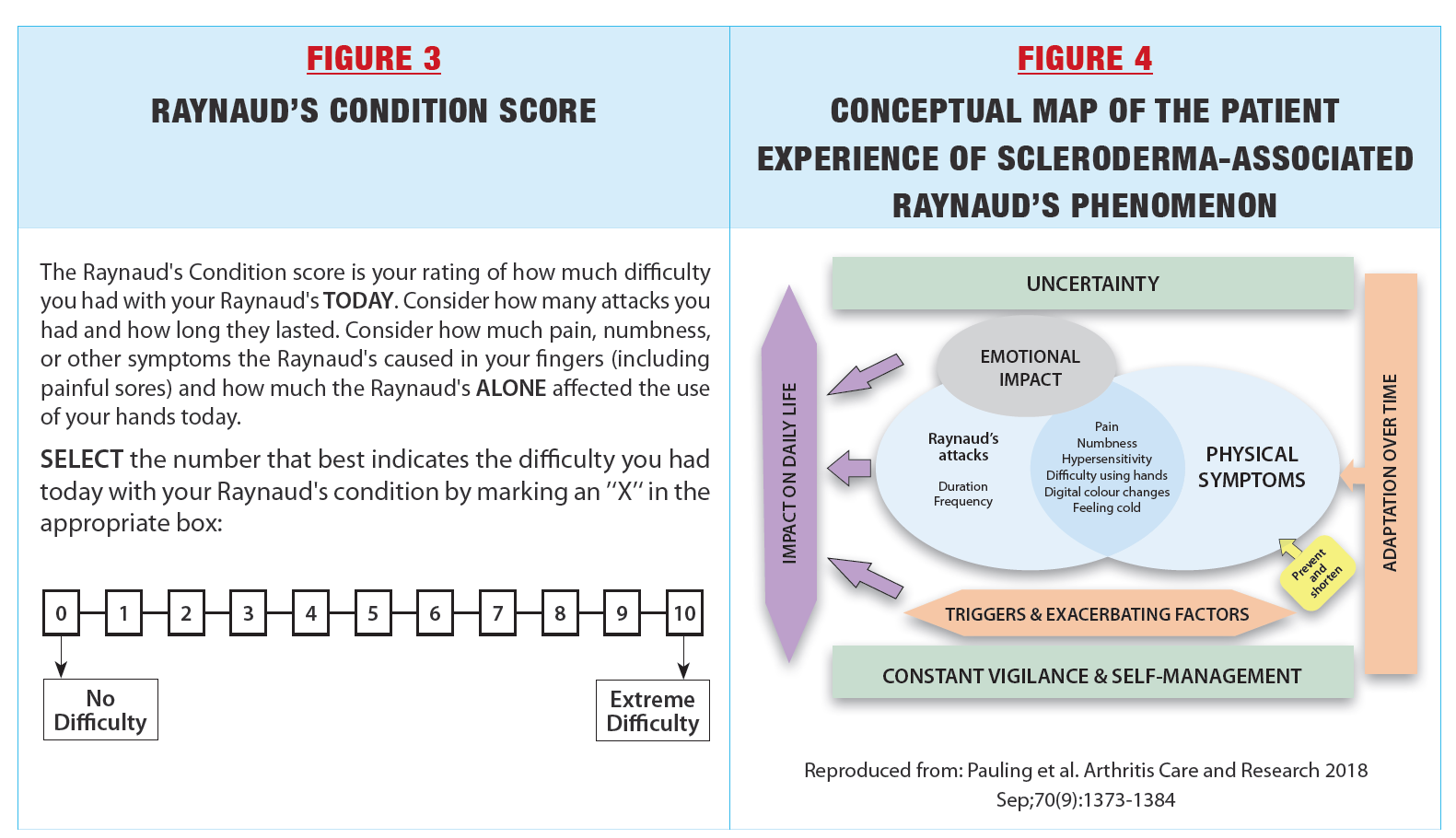
Raynaud’s phenomenon is highly variable and episodic. By its nature, it is one of the symptoms of scleroderma that is best measured by patient reports. In the past, many studies of treatments for Raynaud’s phenomenon used Raynaud’s Condition Score (Figure 3). Patients are asked to rate their Raynaud’s phenomenon from 0–10, taking into account the frequency, duration, pain, numbness, and impact of RP attacks on that day. An average can be taken when the questionnaire is rated on multiple days using a diary. However, several clinical trials of potent vasodilators such as tadalafil, selexipag and bosentan have not shown the expected beneficial results when using Raynaud’s Condition Score, while other PROs such as the HAQ showed improvement. This suggests that the single item Raynaud’s Condition Score does not capture the multifaceted experience of Raynaud’s phenomenon. |
In recent years, there has been remarkable progress in better measuring Raynaud’s phenomenon. Extensive patient input was collected to develop a conceptual framework for capturing the lived experience of how scleroderma patients “feel and function” concerning their Raynaud’s (Figure 4).(9) From this, a novel scleroderma-specific measure of Raynaud’s phenomenon, the Assessment of Systemic sclerosis-associated RAynaud’s Phenomenon (ASRAP) questionnaire, was developed and recently published.(10) It consists of 10 items covering not only frequency and duration of attacks but also facets such as ‘emotional distress’, ‘exacerbating factors’, ‘self-management’ and ‘adaptation’. The ASRAP should provide a more nuanced measure of Raynaud’s phenomenon in scleroderma and provide a useful tool to test new treatments for this often debilitating problem. |
|
 |
 |
 |
||
|
Visible disfigurement especially of the face and hands is common in scleroderma and has been associated with body image dissatisfaction and social discomfort. Body image is inherently personal and PROs have been developed and tested in scleroderma. In particular, the Body Concealment Scale for Scleroderma (BCSS) was developed to capture the unique body image concerns of scleroderma patients (Table 1). (11) Many other PROMs cover a broad range of scleroderma patient experiences including breathlessness, gastrointestinal symptoms and hand function (Table 2). Most of the measures are limited to a single organ and are not specific to scleroderma. The EULAR ScleroID is a novel, disease-specific, composite PROM that was designed to capture the global burden of disease (Figure 5). (12) It consists of 10 items including pain and fatigue. Each of the items is weighted and the final score ranges from 0 to 10, with higher scores indicating worse disease. It is a promising new tool to capture patient experience in future studies of scleroderma. |
Although there has been tremendous progress in measuring PROs in scleroderma in the last 2 decades, the disease-specific instruments developed with extensive patient input, in particular the ASRAP and EUSTAR ScleroID, have yet to be used in clinical trials. Those studies are highly anticipated. IMPORTANCE OF MEASURING PROS PROs provide a unique opportunity to capture the personal experiences of scleroderma patients and add tremendous depth to our understanding of how they “feel and function”, above and beyond standard biomedical measures of disease. PROs are used to identify research priorities of relevance to patients, to advocate for health care resources and for regulatory drug approvals. Although more work on PROs in scleroderma remains, the advances of the last two decades have given a voice to scleroderma patients that can only get stronger with time. |
|
References
1. Kluetz PG, O’Connor DJ, Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol 2018;19(5):e267-e74. doi: 10.1016/S1470-2045(18)30097-4 [published Online First: 2018/05/05]
2. Hudson M, Impens A, Baron M, et al. Discordance between patient and physician assessments of disease severity in systemic sclerosis. J Rheumatol 2010;37(11):2307-12. doi: 10.3899/jrheum.100354 [published Online First: 2010/09/17]
3. Hudson M, Thombs BD, Steele R, et al. Health-related quality of life in systemic sclerosis: a systematic review. Arthritis Rheum 2009;61(8):1112-20. doi: 10.1002/art.24676
4. Hudson M, Thombs BD, Steele R, et al. Quality of life in patients with systemic sclerosis compared to the general population and patients with other chronic conditions. J Rheumatol 2009;36(4):768-72. doi: 10.3899/jrheum.080281
5. Kwakkenbos L, Thombs BD, Khanna D, et al. Performance of the Patient-Reported Outcomes Measurement Information System-29 in scleroderma: a Scleroderma Patient-centered Intervention Network Cohort Study. Rheumatology (Oxford) 2017;56(8):1302-11. doi: 10.1093/rheumatology/kex055 [published Online First: 2017/04/22]
6. Steen VD, Medsger TA, Jr. The value of the Health Assessment Questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum 1997;40(11):1984-91. doi: 10.1002/ art.1780401110 [published Online First: 1997/11/19]
7. Man A, Correa JK, Ziemek J, et al. Development and validation of a patient-reported outcome instrument for skin involvement in patients with systemic sclerosis. Ann Rheum Dis 2017;76(8):1374-80. doi: 10.1136/annrheumdis-2016-210534 [published Online First: 2017/02/19]
8. Man A, Dgetluck N, Conley B, et al. Performance of the scleroderma skin patient-reported outcome (sspro) in a phase 2 trial with lenabasum. Annals of the rheumatic diseases 2019;78:848‐49 (suppl).
9. Pauling JD, Domsic RT, Saketkoo LA, et al. Multinational Qualitative Research Study Exploring the Patient Experience of Raynaud’s Phenomenon in Systemic Sclerosis. Arthritis Care Res (Hoboken) 2018;70(9):1373-84. doi: 10.1002/acr.23475 [published Online First: 2018/02/24]
10. Yu L, Domsic RT, Saketkoo LA, et al. The Assessment of Systemic sclerosis-associated RAynaud’s Phenomenon (ASRAP) questionnaire: Item Bank and Short Form Development. Arthritis Care Res (Hoboken) 2022 doi: 10.1002/acr.25038 [published Online First: 2022/10/11]
11. Jewett LR, Malcarne VL, Kwakkenbos L, et al. Development and Validation of the Body Concealment Scale for Scleroderma. Arthritis Care Res (Hoboken) 2016;68(8):1158-65. doi: 10.1002/acr.22819 [published Online First: 2015/12/15]
12. Becker MO, Dobrota R, Garaiman A, et al. Development and validation of a patient-reported outcome measure for systemic sclerosis: the EULAR Systemic Sclerosis Impact of Disease (ScleroID) questionnaire. Ann Rheum Dis 2022;81(4):507-15. doi: 10.1136/annrheumdis-2021-220702 [published Online First: 2021/11/27]







