Dermatological Interventions for Facial Manifestations in Scleroderma

 |
As a result of scleroderma, many patients experience functional and cosmetic impairment of the face. This may affect the quality of life and psychological health of patients. Unfortunately, despite the increasing use of cosmetic procedures in the general population, there is a lack of evidence evaluating the safety and effectiveness of these procedures in scleroderma patients. In this article, we explore some dermatological interventions used to address common facial manifestations of scleroderma. |
|
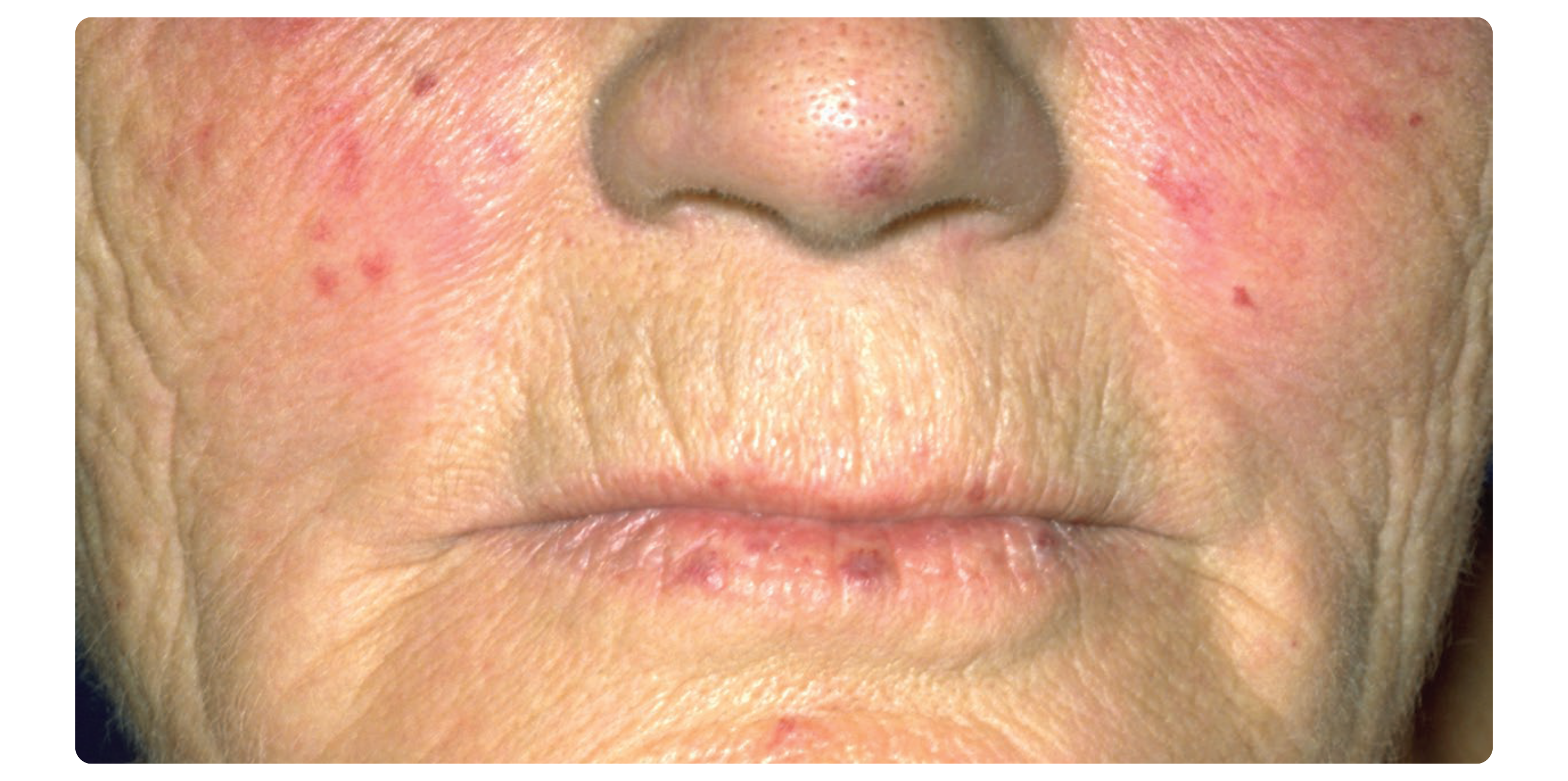
TELANGIECTASIAS Telangiectasias are dilations of small blood vessels that can appear as red spots on the skin in patients with scleroderma. They commonly occur on the face, and less commonly on other sun-exposed areas. Although not dangerous, telangiectasias can be a source of psychological distress and body image dissatisfaction. There are few studies on the treatment of telangiectasias in scleroderma, which are described to be more resistant to treatment than non-scleroderma-related telangiectasias. Although evidence is lacking, there are two promising treatments: pulsed dye laser (PDL) and intense pulse light (IPL), both of which are non-invasive treatments with good safety profiles. PDL (a type of laser) converts a narrow range of light waves into heat to destroy the damaged blood vessels causing telangiectasias while keeping healthy tissues and vessels safe. A retrospective study of 23 scleroderma patients with facial telangiectasias treated monthly with PDL for an average of 3 months showed improvement in all patients, and clearance of telangiectasias in 44% of patients (1). However, 26% of patients had a recurrence of facial telangiectasias after 6-36 months. Common side effects include purple spots (which represent bruising) that appear for 5-10 days after the procedure. Other less common side effects include swelling, self-healing blistering in treated areas, changes in skin pigment colour, and very rarely scarring. All patients in this study stated they would repeat the treatment. IPL (a type of flashlamp emitting visible light) works similarly to PDL. The main difference between IPL and PDL, however, is the type of light being used: IPL uses a broadband pulsed light source, whereas PDL uses a monochromatic coherent light source. A few studies in scleroderma patients have shown that IPL can be effective for facial telangiectasias as well and is well tolerated. One study comparing PDL and IPL in scleroderma patients with facial telangiectasias confirmed that they were both effective treatments, but that PDL was perhaps more effective and that IPL had fewer side effects (2). As IPL emits a broader range of light waves, it may also help to improve skin pigmentary changes (sun or scleroderma-induced) and signs of skin aging through its effect on pigmentary cells and collagen (3). |
While there are no studies that assessed the effect of sun and smoking on the number and severity of scleroderma-associated telangiectasias, telangiectasias in general (including scleroderma-associated) are typically more prominent in skin chronically exposed to ultraviolet light and in patients who are smokers (4,5). As such, it is a common practice for dermatologists and other health care providers to recommend daily sun protection to limit ultraviolet-induced skin damage and prevent skin cancer. Recommendations regarding sun safety can be found on the Canadian Dermatology Association website (https://dermatology.ca/public-patients/ sun-protection/sun-safety-every-day/). Tobacco cessation is prudent to limit scleroderma-associated complications, skin cancer and skin aging. |
MICROSTOMIA
Microstomia is defined as a mouth opening of less than 50 mm, when measured between the top and bottom front teeth (called inter-incisor distance). It is a common feature in scleroderma, affecting up to 80% of patients. Because of the decreased mouth opening, microstomia also impairs eating, dental hygiene, and speaking, and alters facial appearance. Although further research is needed to determine the most effective treatments for microstomia, there are some promising treatment options available for patients who suffer from these negative consequences on their oral and digestive health, as well as body image.
Hyaluronidase is a protein enzyme that breaks down hyaluronic acid, which contributes to skin tightening in scleroderma. Thus, its use is proposed to soften the tight skin in scleroderma. It has only been studied in a few case reports and case series, but the results of these studies and the proposed mechanism of action are promising. It is injected into several regions around the mouth and can be injected monthly for multiple doses, with reported improvements in interincisor distance of up to 15 mm (6). There are several ongoing studies aiming to determine dosing, frequency and number of injections needed to improve the mouth opening. Outside of scleroderma, hyaluronidase has been used routinely for many years in the cosmetic industry and thereby its safety is well established. The main potential side effects of hyaluronidase injection include pain during injection (which can be minimized by using topical analgesia or nerve blocks), bruising and very rarely an allergic reaction.
Other more invasive treatments such as autologous fat grafting have shown promising results as well. Autologous fat grafting involves taking fat from a patient (such as around the hips, knees, or abdomen), and re-injecting it (sometimes mixed with another substance, platelet-rich plasma) around the mouth.
There have been clinical trials, as well as case series and case reports showing varying levels of improvement in the inter-incisor distance following this procedure. However, this procedure is invasive, costly and requires general anesthesia in an operating room setting.
As mentioned above, because IPL emits a broader spectrum of light wavelength and is used to reverse signs of skin aging, it has been explored as a treatment for microstomia. In a small study of 4 patients who received 3-5 monthly IPL treatments in the perioral region, all 4 noted softening of the perioral skin and 3 patients described an improved mouth opening (7). However, the data regarding its efficacy remains very scarce. Other light sources (such as phototherapy or other lasers) and botulinum toxin have either shown limited improvement or have only been studied in a few studies.
 |
PERIORAL WRINKLES AND LIP THINNING Cosmetic procedures such as fillers and botulinum toxin injections to reduce wrinkles and increase lip thickness have become more popular in recent years, with many more providers offering these services – sometimes without sufficient training, which can result in harmful complications. Scleroderma patients who suffer from perioral wrinkles (around the mouth) and lip thinning due to their disease may wonder if these procedures are appropriate for them. Several types of fillers can be injected including hyaluronic acid, calcium hydroxylapatite and poly-L-lactic acid. There is a theoretical risk that hyaluronic acid fillers could worsen or reactivate autoimmune diseases such as scleroderma by increasing local tissue inflammation. Because of this, specialized doctors often refuse to perform this procedure in patients with autoimmune diseases such as scleroderma. However, there are some case reports and series (mostly involving patients with stable scleroderma disease on no immune modifying medication (3)) that have shown positive results following the use of hyaluronic acid filler injections and there have been no published reports of scleroderma relapse following filler injection to our knowledge (8). |
Although it remains controversial, hyaluronic acid filler injections can be considered in scleroderma patients with stable disease and on no immune modifying medication, but their safety needs to be verified in larger and higher quality studies. On the other hand, hyaluronic acid fillers of the Vycross family are not recommended, given case reports of late-onset immune-mediated nodules that appear after these types of injections and that are difficult to treat (8).
Few studies have involved calcium hydroxylapatite and poly-L-lactic acid, thus more studies would be needed to confirm their safety in scleroderma. On a cautionary note, although silicone and paraffin fillers have been banned by the Food and Drug Administration and other regulatory agencies for many years due to their high risk of adverse events (local and systemic complications), they are still available on the black market. For these reasons, and because of case reports of scleroderma development following these procedures, we advise against silicone and paraffin filler injections (9). There have been case reports describing the use of botulinum toxin to treat various facial sequelae of scleroderma or skin aging without any adverse effects. To our knowledge, there are no described cases of adverse events following facial botulinum toxin use in scleroderma.
CONCLUSION
Although more research is required, there are some promising methods of addressing facial telangiectasias and microstomia. The use of botulinum toxin and certain fillers in stable scleroderma on no immunosuppressants may be safe.
REFERENCES :
1. Burillo-Martinez S, Prieto-Barrios M, Velasco-Tamariz V, et al. Case series of pulsed dye laser treatment of telangiectasia in 23 patients with systemic sclerosis. Int J Dermatol 2017;56(8):e165-e67. doi: 10.1111/ijd.13595 [première publication en ligne :20170320]
2. Dinsdale G, Murray A, Moore T, et al. A comparison of intense pulsed light and laser treatment of telangiectases in patients with systemic sclerosis: a within-subject randomized trial. Rheumatology (Oxford) 2014;53(8):1422-30. doi: 10.1093/rheumatology/ keu006 [première publication en ligne : 20140313]
3. Creadore A, Watchmaker J, Maymone MBC, et al. Cosmetic treatment in patients with autoimmune connective tissue diseases: Best practices for patients with morphea/systemic sclerosis. J Am Acad Dermatol 2020;83(2):315-41. doi: 10.1016/ j.jaad.2019.12.081 [première publication en ligne : 20200428]
4. Mekić S, Hamer MA, Wigmann C, et al. Epidemiology and determinants of facial telangiectasia: a cross-sectional study. J Eur Acad Dermatol Venereol 2020;34(4):821-26. doi: 10.1111/jdv.15996 [première publication en ligne : 20191125]
5. Jouvray M, Launay D, Dubucquoi S, et al. Whole-Body Distribution and Clinical Association of Telangiectases in Systemic Sclerosis. JAMA Dermatol 2018;154(7):796-805. doi: 10.1001/jamadermatol.2018.0916
6. Min MS, Goldman N, Mazori DR, et al. Hyaluronidase Injections for Oral Microstomia in Systemic Sclerosis and Mixed Connective Tissue Disease. JAMA Dermatol 2023;159(12):1393-95. doi: 10.1001/jamadermatol.2023.3893
7. Comstedt LR, Svensson A, Troilius A. Improvement of microstomia in scleroderma after intense pulsed light: A case series of four patients. J Cosmet Laser Ther 2012;14(2):102-6. doi: 10.3109/14764172.2012.672744
8. Pieretti G, Rafaniello C, Fraenza F, et al. Hyaluronic acid-based fillers in patients with autoimmune inflammatory diseases. J Cosmet Dermatol 2023;22(9):2420-23. doi: 10.1111/jocd.15751 [première publication en ligne : 20230502]
9. Andreu-Barasoain M, Pinedo-Moraleda F, de la Fuente EG, et al. Systemic sclerosis after silicone injections for facial cosmetic








