Cellular Therapies for Scleroderma

Systemic sclerosis (SSc) is a chronic, systemic autoimmune disease characterized by a pathogenic triad of vasculopathy, immune dysregulation and fibrosis that results in multi-organ dysfunction affecting primarily the skin, gastrointestinal tract, lungs, heart and kidneys. Mortality rates for SSc compared to the general population have remained high (standardized mortality ratios above 3.5) in the past 50 years, in sharp contrast to the significant reductions in mortality in numerous other diseases, including cancer and cardiorespiratory diseases.
In addition to reduced survival, SSc is also notable for significant morbidity that results from Raynaud’s phenomenon, finger ulcers, joint contractures, gastro-esophageal reflux disease, malabsorption, diarrhea, constipation, fecal incontinence, and exertional dyspnea, among others. These translate into limitations in physical mobility and function, disfigurement, pain, fatigue, sleep disturbance, and depression. SSc is associated with significant impairment in health-related quality of life on average 1½ standard deviations below the general population, comparable to or worse than that of patients with other chronic conditions, including heart disease, lung disease, hypertension, diabetes and depression.
SSc remains an orphan disease with high unmet needs in the field of therapeutics. The recommended treatments are mostly symptomatic, with drugs alleviating the symptoms mentioned above rather than targeting the disease as a whole. Also, immunosuppressive drugs such as cyclophosphamide and mycophenolate mofetil have at best modest effects aimed at stabilizing disease, without improving survival.
Similar to lung or kidney transplant where a whole organ is harvested from a healthy donor and transferred to someone with lung or kidney failure, cell therapies involve the harvesting of healthy human cells (whether from a donor or even the patients themselves) which are then transfused into a patient to restore or repair a diseased cell or organ. The best known type of cell therapy is blood transfusions. Some cells used as cell therapies have long-lasting effects and are therefore valued for their regenerative properties.
The purpose of this information brochure is to provide an overview of the cellular therapies presently available or currently under investigation for the treatment of SSc.
HEMATOPOIETIC STEM CELL (HSC) TRANSPLANTATION |
||
|
OVERVIEW
|
|
WHO IS AND IS NOT A CANDIDATE FOR HSC TRANSPLANT FOR SCLERODERMA? Because of the toxicity associated with the transplant itself, HSC transplant is not for everyone. Patients are carefully selected. The main selection criteria are the following: MAIN INDICATIONS
MAIN CONTRAINDICATIONS
|
WHAT ARE THE STEPS FOR A HSC TRANSPLANT? |
||
|
|
||
|
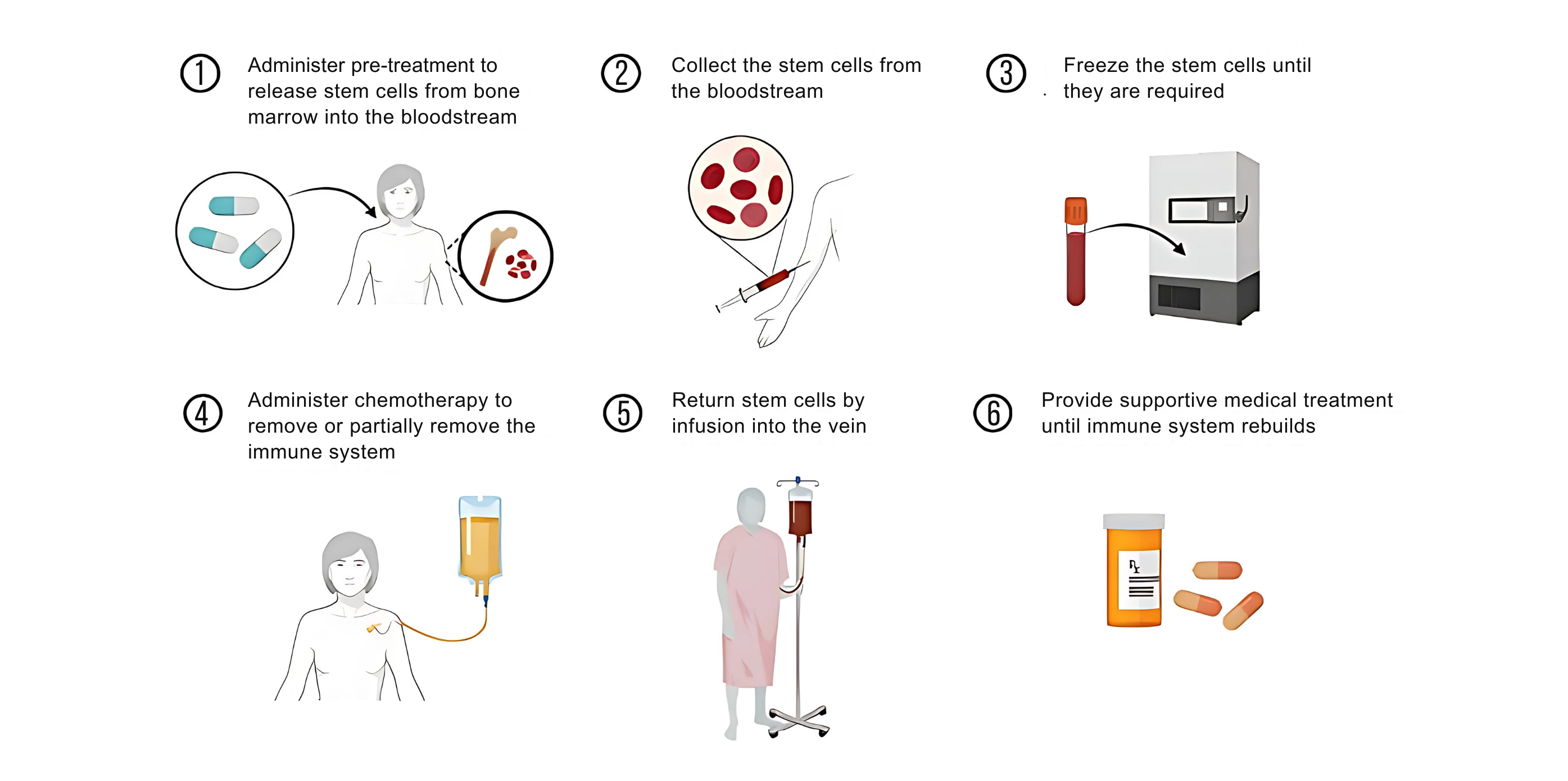
STEP 1: MOBILIZATION (A FEW DAYS)
|
STEP 4: PRE-TRANSPLANT TREATMENT (I.E., CONDITIONING, 5-10 DAYS) High dose chemotherapy, and sometimes radiotherapy, is given to eliminate your unhealthy immune cells. |
|
|
STEP 2: STEM CELL COLLECTION (1 DAY)
|
STEP 5: GETTING YOUR STEM CELLS BACK (I.E. HSC TRANSPLANT, AROUND 30 MINUTES) Your own stem cells that were previously collected and stored are infused back into you through an IV. |
|
| STEP 3: STORAGE The stem cells are stored in a cell therapy laboratory until they are needed. |
STEP 6: RECOVERY Close monitoring is required for weeks in the hospital and then months as an out-patient until your stem cells recover their normal function and regenerate a healthy immune system. |
|
WHAT TO CONSIDER BEFORE A TRANSPLANT?
Studies have shown that the most common questions from scleroderma patients considering HSC transplant were the following:
- Will I be supported by a multidisciplinary team?
- What are the financial risks?
- Where can I get reliable information about HSC transplant?
- Are there physical risks associated with HSC transplant?
- What are the benefits of HSC transplant for someone in my condition?
Here are some resources to help with answering some of these important questions:
www.astemcelljourney.com
https://mathec.com
WHAT TO EXPECT AFTER TRANSPLANT?
HSC transplant is not a cure for scleroderma and the damage that has occurred prior to transplant is generally not reversible. However, HSC transplant is currently the best treatment to prevent disease progression and the only one shown to increase survival in scleroderma. Also, people with scleroderma have much better function and quality of life after HSC transplant compared to those treated with standard treatments.
MESENCHYMAL STROMAL CELLS (MSCS)
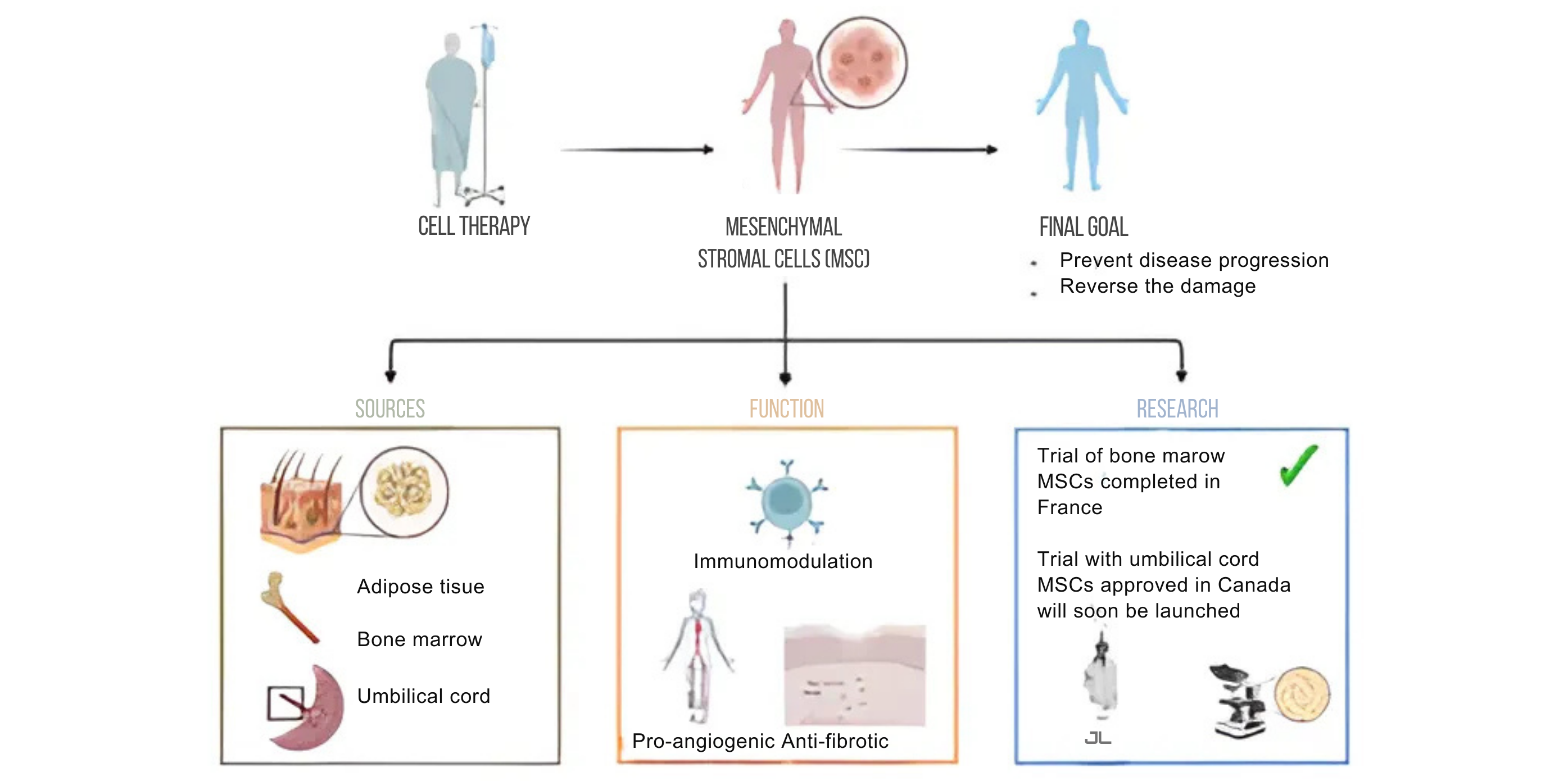
Because HSC transplant can be toxic and is not for everyone, different types of cellular therapies are being investigated. Mesenchymal stromal cells (MSCs) have immunomodulatory, pro-angiogenic, and anti-fibrotic properties and therefore have the potential to target all three axes of the SSc pathogenic triad. MSCs are present in nearly every tissue. They are responsible for keeping those tissues healthy. For therapeutic uses, they are usually harvested from bone marrow, adipose tissue, and umbilical cord. Injections of MSCs have been studied in a wide range of diseases, including other autoimmune diseases, and have been shown, first, to be safe (in large part because they do not need to be administered with chemotherapy) and second, to help in tissue repair and regeneration.
In scleroderma, MSCs are thought to work by re-setting the diseased immune system. This in turn can lead to less fibrosis and better circulation. To date, an early trial of bone marrow derived MSCs has been completed in France and results are promising. However, harvesting MSCs from the bone marrow is fairly invasive. Umbilical cords represent a promising alternative. A study of umbilical cord derived MSCs in scleroderma has been approved in Canada and should begin soon.
Many questions remain around the benefits of MSCs for scleroderma. What is the best source of MSCs? How can the function of MSCs from different donors be standardized? Will the benefits be sustained or will repeated infusions be required? If so, can MSCs from different sources and donors be used? In addition to infusions through the veins, can MSCs be injected locally, for example in the fingers, face or muscles? Can MSCs be produced in sufficient quantities to meet the demands? Instead of whole cells, are there specific components of MSCs or alternative cell products that could be more effective and more easily manufactured? And the list goes on!
CONCLUSION
Research is key to answering these and other questions. Identifying new, safe and accessible therapies that not only prevent disease progression but ideally reverse the damage that has already accumulated should remain the top priority for scleroderma research.
ADDITIONAL RESOURCES:
- Richard K. Burt, Dominique Farge, Milton A. Ruiz, Riccardo Saccardi, John A. Snowden (Editors).
- Hematopoietic Stem Cell Transplantation and Cellular Therapies for Autoimmune Diseases. CRC Press; 1st edition (November 15, 2021). ISBN-13: 978-1138558557. ISBN-10: 1138558559.